Asteraceae
Sunflowers, daisies
Jose L. Panero and Bonnie S. Crozier

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Asteraceae (Compositae, alternate name) with its approximately 1,620 genera and more than 23,600 species is the largest family of flowering plants (Stevens, 2001). The family is distributed worldwide except for Antarctica but is especially diverse in the tropical and subtropical regions of North America, the Andes, eastern Brazil, southern Africa, the Mediterranean region, central Asia, and southwestern China. The majority of Asteraceae species are herbaceous, yet an important component of the family is constituted by shrubs or even trees occurring primarily in the tropical regions of North and South America, Africa and Madagascar and on isolated islands in the Atlantic and Pacific Oceans. Many species of sunflowers are ruderal and especially abundant in disturbed areas, but a significant number of them, especially in mountainous tropical regions, are narrow endemics. Because of the relentless habitat transformation precipitated by human expansion in montane tropical regions, a number of these species are consequently in danger of extinction.
The family contains several species that are important sources of cooking oils, sweetening agents, and tea infusions. Members of several genera of the family are well-known for their horticultural value and popular in gardens across the world and include zinnias, marigolds, dahlias, and chrysanthemums. The commercial sunflower genus Helianthus has been used as a model in the study of hybridization and its role in speciation (Rieseberg et al., 2003). See list of economically important Asteraceae
Characteristics
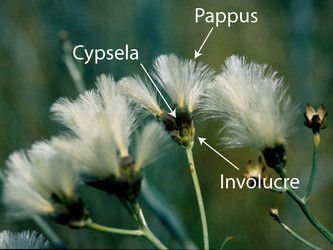
The family is characterized by having a capitulum or head, an inferior, unilocular ovary with one ovule, and with few exceptions fused anthers surrounding the style. The capitulum (capitula plural) is a specialized indeterminate inflorescence that can contain 1 to hundreds of individual flowers (florets). The flowering sequence in the capitulum is nearly always from the outside to the center, that is, centripetal (Harris, 1995). The florets sit on the disc or receptacle, an expanded shoot that can be flat, concave, convex, or rarely columnar. The disc and florets are surrounded by bracts or leaf-like structures called phyllaries and collectively forming an involucre. The phyllaries can be arranged in one row and be of equivalent length or can be unequal in length. Most sunflowers have involucres with several series of phyllaries. In these sunflowers the phyllaries can be subequal with all phyllaries of equivalent length or imbricate. Involucres composed of imbricate phyllaries are the most common condition in the family and exemplified by the artichoke (Cynara cardunculus) in which multiple series of phyllaries overlap each other. In some species the outermost phyllaries sometimes resemble leaves. The receptacle can be naked or sometimes have bract-like structures called paleae, scales or hairs surrounding each floret. The involucre can have different shapes ranging from tubular to hemispheric.
There are six types of corollas present in the Asteraceae two of which are actinomorphic and the other four are zygomorphic (Bremer, 1994). Actinomorphic corollas are composed of five equivalent lobes and normally termed disc corollas (as they occupy most of the disc area). They have five lobes and when viewed from above, the reflexed lobes of the actinomorphic corolla resemble a five point star. Disc corollas may have fewer lobes with four being the most common departure, although corollas with three lobes are also seen. Tubular corollas are narrow actinomorphic corollas, mostly lacking stamens. Zygomorphic corollas are mostly confined to the first row of florets in the capitulum, although some species may have several rows of zygomorphic corollas. Bilabiate corollas are generally present only in several genera belonging to the earliest divergences of the family. The bilabiate corolla has a 3+2 arrangement of lobes with the 3-lobe lamina facing towards the outside and the 2-lobe lamina to the center of the capitulum. Sometimes the 2 lobes are separate and coiled. The pseudobilabiate corolla has a 4+1 arrangement. The ray floret is present in several tribes of the subfamilies Cichorioideae and Asteroideae and consists of a lamina that terminates in 2-3 lobes. Some members of tribe Arctotideae have a ray floret that terminates in 4 lobes. Ligulate corollas have 5 lobes.
The capitula of sunflowers can contain florets with corollas of the same morphology or a combination of two or sometimes three types of corollas. In discoid capitula all florets have actinomorphic corollas and can be either bisexual and fertile or functionally staminate or pistillate. In radiate capitula the peripheral florets are ray florets and these can be pistillate or styliferous and sterile or neuter (no style present). Radiant capitula are discoid capitula with peripheral corollas having lobes variously expanded. In ligulate or liguliflorous capitula all the florets are bisexual and have ligulate corollas; this capitulum type is only found in members of tribe Cichorieae. Disciform capitula have florets with actinomorphic corollas with peripheral florets having tubular corollas. These peripheral corollas are pistillate. Homogamous capitula have florets exhibiting similar sexual forms whereas heterogamous capitula have florets with two or more sexual forms.
Most sunflower florets have five anthers corresponding to the number of lobes in the corolla. The anthers are positioned along the sinuses of the corolla lobes (alternate to the lobes). The anther filaments are free from the corolla just above the tube and the two thecae (pollen sacs) of each stamen are connate with the thecae of adjacent stamens producing a tube that surrounds the style. Pollen is shed to the interior of this tube (introrse dehiscence). The connective, the tissue connecting the two anther thecae of each anther, may continue beyond the anther thecae and produce an appendage. Some sunflowers do not have appendages (e.g., many Asteroideae: Eupatorieae). The anther collar is located at the end of the filament just below the connective and is an area of sclerified cells involved in the mechanical aspects of pollen presentation in sunflowers. The anther collar is progressively shorter on the adaxial side facing the style. If the thecae extend below the point of insertion between the filament and connective, the anther is calcarate; if not, the anther is ecalcarate. Anthers can have tails, sometimes very elaborate with branched projections. Calcarate and caudate (tailed) anthers are common among the basal lineages of the family whereas ecalcarate anthers are more common in the Asteroideae.
The styles of sunflowers have two stigmatic branches or arms. They may be smooth (glabrous) or pubescent with trichomes confined mostly to the distal end of the style and the abaxial surfaces of the style branches. In fertile florets, the adaxial surface of the style branch has stigmatic (fertile) papillae. In some groups the stigmatic papillae are continuous throughout the style branches, whereas in others the stigmatic papillae are confined to the margins of the style branches. As the floret matures, the style grows through the tube formed by the fused anthers pushing the pollen up. This secondary pollen presentation mechanism ensures pollen is available to insects visiting the capitulum on a daily basis as new florets open. The pollination biology of sunflowers has not been extensively studied but an excellent summary of the available literature is provided by Lane (1996).
Sunflowers produce dry, indehiscent fruits termed cypselae (achenes in many publications). A few species have fleshy fruits reminiscent of a drupe (Chrysanthemoides, Tilesia). Most species have a pappus on the distal end of the cypsela. This structure is derived from the calyx of the floret (Carlquist, 1976). The pappus aids in dispersal or defense against herbivory (Stuessy and Garver, 1996).
For additional general information concerning the morphology of sunflowers the reader is advised to consult the introductory chapter for the family in Flora North America (Barkley et al., 2006).
Taxonomy
The initial classification of the family was produced by Cassini (1819a,b) who grouped genera into tribes. His tribal concepts have been refined by taxonomists subsequently and are still the main category above the genus level used to classify sunflowers. The grouping of these tribes into subfamilies is a relatively recent academic pursuit in Asteraceae systematics that began with Carlquist (1976) and Wagenitz (1976) defining the concepts of subfamilies Cichorioideae and Asteroideae. The morphological characteristics used to circumscribe these two groups are mostly based on discontinuities in corolla, anther, and style morphology. According to Bremer (1994), the Asteroideae are characterized (with some exceptions) by having true ray florets, disc corollas with short lobes, caveate pollen, stigmatic surfaces of style branches separated into two marginal lines sometimes confluent at apices, and a distinctive secondary chemistry. These morphological characteristics are rarely seen in Cichorioideae.
With the incorporation of results from molecular phylogenetic studies the classification of the Asteraceae has changed relatively quickly, mainly through the recognition of monophyletic groups traditionally included in Cichorioideae. With the work of Jansen and Palmer (1987), Bremer (1994) recognized three subfamilies (Asteroideae, Barnadesioideae, and Cichorioideae) and 17 tribes. Thorne and Reveal (2007) recognized the same subfamily groups using the possibly earlier name Carduoideae for the Cichorioideae. They expanded the number of tribes to 25, recognizing the three new tribes identified by molecular analysis named in Baldwin et al. (2002) but maintained a polypheletic Heliantheae. Jeffrey (2007) accepted the tribal groupings of Panero and Funk (2002), ultimately recognizing 24 tribes and grouping them into five subfamilies. He recognized a monophyletic Barnadesioideae, a monophyletic Asteroideae, and split the grade of clades between these two groups (Cichorioideae of Bremer (1994) or Carduoideae of Thorne and Reveal (2007)) into 3 subfamilies: Mutisioideae, Carduoideae, and Cichorioideae, each shown to be polyphyletic (Panero and Funk, 2002, 2008). As the capacity to expand both taxon and character sampling in molecular studies has grown, providing more resolution and certainty in phylogenetic analyses, more major lineages of the family have been identified. Increased sampling, particularly sampling of taxa considered anomalous in the family (uncertain tribal position, Bremer, 1994), resulted in the discovery that the genera Corymbium, Gymnarrhena, and Hecastocleis represent monotypic lineages sister to major clades and provided evidence for more lineages than previously recognized. The classification of Panero and Funk (2008), shown in the tree above, recognizes 12 strictly monophyletic subfamilies.
The classification of the Asteraceae is dominated by the large subfamily Asteroideae that contains more than 70% of the species of the family. The three main lineages within Asteroideae found by molecular studies (Kim and Jansen, 1995, Panero and Funk, 2008) have been recognized at the supertribe level recently (Robinson, 2004, 2005) as Asterodae, Helianthodae, and Senecionodae. Other large subfamilies include Carduoideae and Cichorioideae each with more than 2000 species. All other subfamilies each contain less than 1000 species with Gymnarrhenoideae and Hecastocleidoideae containing only one species each.
Discussion of Phylogenetic Relationships
The phylogenetic tree shown on this and subsequent pages is drawn after that reported in Panero and Funk (2008) based on analyses of combined sequence data from multiple chloroplast loci including the genes matK, ndhD, ndhI, ndhF, rbcL, rpoB, exon1 of rpoC1, and the intron and intergenic spacer regions of the trnL-trnF. Each of the major clades of Asteraceae (subfamilial and tribal clades) was inferred in both Maximum Parsimony and Bayesian phylogenetic analyses with significant measures of clade support (bootstrap and posterior probability respectively). Relationships among these clades were also resolved with significant statistical support and were robust to method with the exception of the relationships of Stifftioideae and Wunderlichioideae. The phylogeny of Panero and Funk (2008) represents the best resolved and statistically supported hypothesis of evolutionary relationships among the major clades of the family currently available. Preliminary results from their study (Panero and Funk, 2008) have been used previously as the backbone phylogeny for the construction of a metatree of the family (Funk et al., 2009). The nomenclature used here is consistent with that of NCBI Genbank.
Relationships among the deepest branches of the Asteraceae tree of life have primarily been elucidated through investigations of the chloroplast genome. Comparative nucleotide studies (Kim and Jansen, 1995, Bayer and Starr, 1998, Panero and Funk, 2008) confirmed the original discovery based on restriction fragment polymorphisms (RFLPs) that Barnadesioideae, lacking the 22 kb inversion present in all other sunflowers (Jansen and Palmer, 1987), is sister to all other Asteraceae. A Cichorioideae clade sister to Asteroideae was recovered with weak bootstrap support in the ndhF study of Kim and Jansen (1995) and identified with strong support (bootstrap and posterior probability) in the multi-locus chloroplast study of Panero and Funk (2008). The multi-locus study also recovered strong statistical support for the Mutisioideae, Stifftioideae, Gochnatioideae, Hecastocleidoideae, Carduoideae, Pertyoideae, Gymnarrhenoideae, and Corymbioideae clades. All family-wide molecular phylogenetic studies to date (Kim and Jansen, 1995, Bayer and Starr, 1998, Kim et al., 2005, Panero and Funk, 2008) have shown support for a monophyletic Asteroideae.
Evidence from the nuclear genetic compartment, an analysis of the nuclear ribosomal internal transcribed spacer (nrITS) region (Goertzen et al., 2003), is congruent with chloroplast results in the relationships of Barnadesioideae and Mutisioideae to other Asteraceae, in the monophyly of the Asteroideae, and in the sister relationship of Athroismeae and Heliantheae s.l. Relationships of other major clades of Asteraceae recovered from the nrITS study (strict consensus tree) are not congruent with chloroplast results. However, these may conservatively be viewed as equivocal because of low statistical support (bootstrap proportions).
The phylogenetic tree presented here primarily reflects the evolutionary history of the chloroplast genome. Asteraceae provide some of the best documented examples of hybrid evolution in plants (Rieseberg et al., 2003, Soltis et al. 2008, Timme et al., 2007), but the full extent of reticulation in the evolutionary history of sunflowers is not yet known, nor how closely this genome phylogeny corresponds to the organismal phylogeny of sunflowers.
Fossil Record
The fossil record of Asteraceae is for the most part composed of pollen deposits and fruits. Few pollen records exist for the Eocene but the pollen of Asteraceae becomes increasingly common in samples dated to the Oligocene/Miocene (Graham, 1996). These data show the increasing importance of the family in most biomes of the world from the mid to late Oligocene to present. Pollen samples from Paleocene-Eocene deposits in southwestern Africa have been attributed to Mutisieae (Zavada and De Villiers, 2000; De Villiers and Cadman, 2001) or to a Dicoma-like taxon and recently dated to the mid-Eocene (Scott et al., 2006). Asteraceae pollen dated to the Eocene has also been found in Egypt (Kedves, 1971), China (Song et al., 1999) and North America (Texas Gulf coast, Elsik and Yancey, 2000).
Fossil pollen records have been used recently to shed light on the time of origin of Asteraceae. Dated and properly identified, pollen deposits can be used to place a minimum age for the origin of a particular clade. Using the rate of mutation of the gene rbcL and a fossil calibration, Bremer and Gustafsson (1997) concluded the family to have originated at least 38 Ma. A similar approach using the ndhF and rbcL genes was used by Kim et al. (2005) to date the origin of molecular rearrangements in the chloroplast genome of the Asteraceae. Their study concluded that the family originated in the mid Eocene (42-47 Ma).
References
Barkley, T.M., L. Brouillet, and J. L. Strother. 2006. Asteraceae. Pages 3-69 in Flora of North America, vol. 19. Oxford University Press, New York.
Bayer, R. J. and J. R. Starr. 1998. Tribal phylogeny of the Asteraceae based on two noncoding chloroplast sequences, the trnL intron and the trnL-F intergenic spacer. Ann. Missouri Bot. Gard. 85: 242-256.
Bremer, K. 1994. Asteraceae: Cladistics and Classification. Timber Press, Portland, Oregon.
Bremer, K., and M. H. G. Gustafsson. 1997. East Gondwana ancestry of the sunflower alliance of families. Proc. Natl. Acad. Sci. USA. 94: 9188-9190.
Carlquist, S. 1976. Tribal interrelationships and phylogeny of the Asteraceae. Aliso 8: 465-492.
Cassini, H. 1819a. Sexième mémoire sur la famille des Synanthérées, contenant les caractères des tribus. J. Phys. Chim. Hist. Nat. Arts 88: 150-163.
Cassini, H. 1819b. Suite du sexième mémoire sur la famille des Synanthérées, contenant les caractères des tribus. J. Phys. Chim. Hist. Nat. Arts 88: 189-204.
De Villiers, S. E., and A. Cadman. 2001. An analysis of the palynomorphs obtained from Tertiary sediments at Koingnaas, Namaqualand, South Africa. J. Afr. Earth Sci. 33: 17-47.
Elsik, W. C., and T. E. Yancey. 2000. Palynomorph biozones in the context of changing paleoclimate, middle Eocene to lower Oligocene of the Northwest Gulf of Mexico. Palynology 24: 177-186.
Funk, V. A ., R. J. Bayer, S. Keeley, R. Chan, L. Watson, B. Gemeinholzer, E. E. Schilling, J. L. Panero, B. G. Baldwin, N. T. García Jacas, A. Susanna, and R. K. Jansen. 2005. Everywhere but Antarctica: using a supertree to understand the diversity and distribution of the Compositae. Pages 343-373 in Plant diversity and complexity patterns-local, regional and global dimensions, (I. Friis and H. Balslev, eds.). Biol. Skr. 55.
Goertzen, L. R., J. J. Cannone, R. R. Gutell, and R.K. Jansen. 2003. ITS secondary structure derived from comparative analysis: implications for sequence alignment and phylogeny of Asteraceae. Mol. Phylogenet. Evol. 29: 216-234.
Graham, A. 1996. A contribution to the geologic history of the Compositae. Pages 123-140 in Compositae: Systematics. Proceedings of the International Compositae Conference, Kew, 1994, (D. J. N. Hind and H. Beentje, H. J. eds.). Royal Botanic Gardens, Kew.
Harris, E. M. 1995. Inflorescence and floral ontogeny in Asteraceae: a synthesis of historical and current concepts. Botanical Review 61: 93-278.
Jansen, R. K., and J. D. Palmer. 1987. A chloroplast DNA inversion marks an ancient evolutionary split in the sunflower family (Asteraceae). Proc. Natl. Acad. Sci. U.S.A. 84: 5818-5822.
Jeffrey, C. 2007. Compositae: Introduction with key to tribes. Pages 61-87 in Families and Genera of Vascular Plants, vol. VIII, Flowering Plants, Eudicots, Asterales (J. W. Kadereit and C. Jeffrey, eds.). Springer-Verlag, Berlin.
Kedves, M. 1971. Presence de types sporomorphs importants dans les sediments prequaternaires Egyptiens. Acta. Bot. Hung. 17: 371-378.
Kim K.-J., R. K. Jansen. 1995. ndhF sequence evolution and the major clades in the sunflower family. Proc. Natl. Acad. Sci. USA 92: 10379-10383.
Kim K.-J., K. S. Choi, and R. K. Jansen. 2005. Two chloroplast DNA inversions originated simultaneously during the early evolution of the sunflower family (Asteraceae). Mol. Biol. Evol. 22: 1783-1792.
Lane, M., 1996. Pollination biology of Compositae. Pages 61-80 in Compositae: Biology and Utilization, Proceedings of the International Compositae Conference, Kew, 1994, (P. D. S. Caligari and D. J. N. Hind, eds.). Royal Botanic Gardens, Kew.
Panero, J. L., and V. A. Funk. 2002. Toward a phylogenetic subfamilial classification for the Compositae. Proc. Biol. Soc. Wash. 115: 909-922.
Panero, J. L., and V. A. Funk. 2008. The value of sampling anomalous taxa in phylogenetic studies: major clades of the Asteraceae revealed. Mol. Phylogenet. Evol. 47: 757-782.
Rieseberg, L. H., O. Raymond, D. M. Rosenthal, Z. Lai, K. Livingstone, T. Nakazato, J. L. Durphy, A. E. Schwarzbach, L. A. Donovan, C. Lexer. 2003. Major ecological transitions in wild sunflowers facilitated by hybridization. Science 301: 1211-1216.
Robinson, H. 2004. New supertribes Helianthodae and Senecionodae, for the subfamily Asteroideae (Asteraceae). Phytologia 86: 116-120.
Robinson, H. 2005. Validation of the supertribe Asterodae. Phytologia 87: 72-88.
Scott, L., A. Cadman, and I. McMillan. 2006. Early history of Cainozoic Asteraceae along the southern African west coast. Rev. Palaeobot. Palyno. 142: 47-52.
Soltis, D. E., E. V. Mavrodiev, J. J. Doyle, J. Rauscher, and P. S. Soltis. 2008. ITS and ETS sequence data and phylogeny reconstruction in allopolyploids and hybrids. Syst. Bot. 33: 7-20.
Song, Z. C., Y. H. Zheng, and M. Y. Li. 1999. Paleogene palynostratigraphy. Pages 141-265 in Fossil Spores and Pollen of China: The Late Cretaceous and Tertiary Spores and Pollen (Z. C. Song, Y. H. Zheng, M. Y. Li, Y. Y. Zhang, W. M. Wang, D. N. Wang, C. B. Zhao, S. F. Zhou, Z. H. Zhu, and Y. N. Zhao, eds.). Science Press, Beijing.
Stevens, P. F. (2001 onwards). Angiosperm Phylogeny Website. Version 8, June 2007 [and more or less continuously updated since]. http://www.mobot.org/MOBOT/research/APweb/. (Accessed March 27, 2008).
Stuessy, T.F., and D. Garver. 1996. The defensive role of pappus in heads of Compositae. Pages 81-91 in Compositae: Biology and Utilization, Proceedings of the International Compositae Conference, Kew, 1994, (P. D. S. Caligari and D. J. N. Hind, eds.). Royal Botanic Gardens, Kew.
Timme, R. E., B. B. Simpson, and C. R. Linder. 2007. High-resolution phylogeny for Helianthus (Asteraceae) using the 18S-26S ribosomal DNA external transcribed spacer. Am. J. Bot. 94: 1837-1852.
Thorne, R. F., and J. L. Reveal. 2007. An updated classification of the class Magnoliopsida ("Angiospermae"). Bot. Rev. 73: 67-181.
Wagenitz, G., 1976. Systematics and phylogeny of the Compositae (Asteraceae). Pl. Syst. Evol. 125: 29-46.
Zavada, M.C., and S. E. De Villiers. 2000. Pollen of Asteraceae from the Paleocene-Eocene of South Africa. Grana 39: 39-45.
Information on the Internet
- Compositae Genome Project Database. Data and information on the Compositae Genome Project.
- Genbank Asteraceae. Database of sunflower DNA sequences. Includes links to other taxonomic/phylogenetic resources.
- Index to chromosome numbers in Asteraceae.
- The International Compositae Alliance. Research news, events, and a list of people studying or interested in sunflowers.
- USDA Germplasm Resources Information Network. USA Government website containing information on taxonomy and germplasm resources of Asteraceae.
Title Illustrations

| Scientific Name | Carduus nutans |
|---|---|
| Location | Saltfleetby NNR, Lincolnshire, UK |
| Specimen Condition | Live Specimen |
| Source | Musk Thistle (Carduus nutans) |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0.
|
| Copyright | © 2007 Les |
| Scientific Name | Werneria nubigena |
|---|---|
| Location | Department of Cusco, Peru |
| Specimen Condition | Live Specimen |
| Source | Werneria nubigena |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0.
|
| Copyright | © 2006 Eric in SF |
| Scientific Name | Chuquiraga jussieui |
|---|---|
| Location | Photographed in situ in the paramo south of Cuenca, Ecuador |
| Specimen Condition | Live Specimen |
| Source | Chuquiraga jussieui |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0.
|
| Copyright | © 2005 Eric in SF |
About This Page
Jose L. Panero

University of Texas, Austin, Texas, USA
Correspondence regarding this page should be directed to Jose L. Panero at
Page copyright © 2012 Jose L. Panero and
 Page: Tree of Life
Asteraceae. Sunflowers, daisies.
Authored by
Jose L. Panero and Bonnie S. Crozier.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Asteraceae. Sunflowers, daisies.
Authored by
Jose L. Panero and Bonnie S. Crozier.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 20 July 2004
- Content changed 27 January 2012
Citing this page:
Panero, Jose L. and Bonnie S. Crozier. 2012. Asteraceae. Sunflowers, daisies. Version 27 January 2012. http://tolweb.org/Asteraceae/20780/2012.01.27 in The Tree of Life Web Project, http://tolweb.org/












 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site