Arcellinida
Testate Lobose Amoebae
Ralf Meisterfeld and Edward Mitchell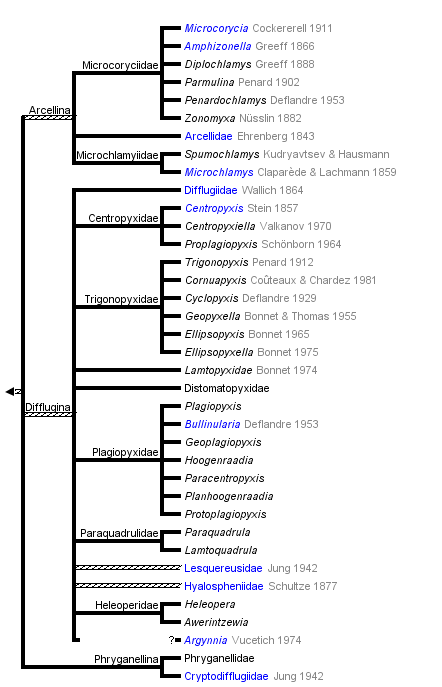

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxThe tree is based on the classification in Meisterfeld, 2002.
Introduction
The Arcellinida are the largest group of the testate amoebae (shelled amoebae). They contain about three quarters of the known species (about 1100 species). But this is only a rough estimate because large parts of the world are only insufficiently sampled.
All Arcellinida are free-living and move and feed with lobopodia. The smallest (Cryptodifflugia) are around 10 µm long while the largest can reach 450 µm. They have a test or tectum which is built in very different ways, and as a result the diversity of life forms is large. Arcellinida are common in all freshwater habitats, inclusive of sediments, periphyton (Aufwuchs), plankton, mosses but also soils. Each habitat type is characterized by specific communities. Testate amoebae are an important component in aquatic and terrestrial ecosystems. They are consumers of bacteria, fungi and different algae groups and are thus important for the energy flow and nutrient cycles in these ecosystems (Aoki, 2007; Meisterfeld, 1989, Schröter et al., 2003).
Characteristics
Shell Morphology
The architecture and composition of the shell has been central in the taxonomy of all shelled amoebae whether lobose or filose (e.g. Euglyphida). Three principle types are common: proteinaceous (organic), agglutinate and tests built with inorganic components synthesized by the cell. Those with proteinaceous test can be divided into
- families which have a more or less flexible membrane that encloses (Microcoryciidae) the cytoplasm
- those with a rigid sheet of fibrous material (some Hyalospheniidae). In this group transitions to agglutinate tests can often be observed (e.g. Nebela)
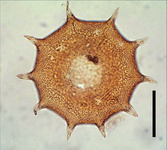
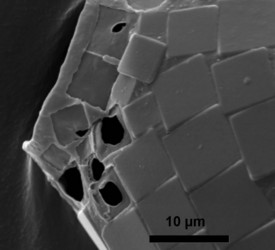
- those with a test constructed of regularly arranged hollow building units to form an areolate surface (Microchlamyiidae, Arcellidae) (Fig. 1).

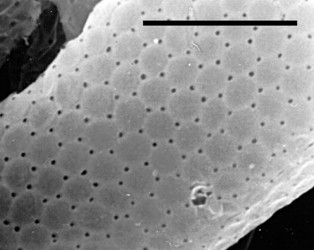
Fig. 1: Shell surface of Arcella hemisphaerica showing the organic building units. © Ralf Meisterfeld
The majority of Arcellinida species have agglutinate tests. In these shells foreign materials like small sand grains or diatoms (xenosomes) are incorporated in an organic cement (Fig. 2). This cement is either a matrix of often perforated building units (Fig. 3), or sheet-like (Fig. 4). The morphology of this cement is of importance for the classification of testate amoebae (Ogden, 1990). A few genera (e.g. Quadrulella, Lesquereusia) have siliceous tests (Fig. 4, 5) composed of rods, nails or rectangular plates which are produced by the organisms themselves (idiosomes).
Calcareous shells are characteristic for the Paraquadrulidae, and the genus Cryptodifflugia. The former has rectangular calcite plates bound by an internal sheet of cement, while in the latter a thick layer of calcium phosphate is deposited within an organic template.
Genera and species are mainly distinguished using differences in shell shape and dimensions. The Arcellinida display a great diversity of shell shapes. Especially in drier habitats such as mosses and soil, the apertural apparatus has evolved to a slit-like structure often covered by the anterior lip (Plagiopyxis) (Fig. 6) or a visor (Planhoogenraadia). The pseudostome (aperture) can be protected by teeth or it can be located at the end of a tubus (tubular section of test leading inwards from aperture e.g. Cyclopyxis puteus).


Fig. 6: Plagiopyxis declivis a typical soil testate amoeba with a slit-like aperture and a flat ventral side. © Ralf Meisterfeld
Cell Morphology
For many species and genera of testate amoebae, few if any details are known about the cytoplasm because they have a thick, opaque shell which prevents or hinders study by light or transmission electron microscopy. Common to the species studied is a zonation of the cytoplasm in an anterior part, close to the aperture with numerous food and digestive vacuoles, and a posterior denser zone, which stains with basic dyes and contains usually one, rarely two or more nuclei (genus Arcella). This region is a dense rough ER with one or more dictyosomes within or more often at the periphery. The Golgi complex is involved in the secretion of organic building units and cement and perhaps in the synthesis of siliceous idiosomes (e.g. Harrison et al., 1976, 1981). Contractile vacuoles are located lateral to this region. Mitochondria are usually ovoid or spherical and have branched tubular cristae. They are concentrated in the posterior part of the cell.
 Fig. 7: Vesicular nuclei of Arcella vulgaris multinucleata. n= nucleus, ncl= nucleolus. © Ralf Meisterfeld | 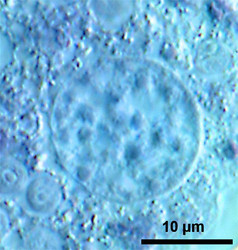 Fig. 8: Ovular nucleus of Hyalosphenia papilio. © Ralf Meisterfeld |
According to the classification of Raikov (1982) two principle types of nuclei are found in testate amoebae: 1. vesicular nuclei with one, often central nucleolus (Raikov et al. 1989), sometimes with a few additional very small nucleoli (Fig. 7); 2. ovular nuclei with several to many small nucleoli (Fig. 8).
Small vesicles of cement are usually scattered throughout the cytoplasm in genera with the test constructed of hollow, organic building units (Netzel, 1975; Mignot & Raikov, 1990).
Pseudopodia

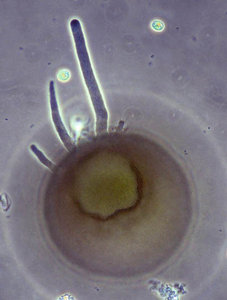

Fig. 9: Endolobopodia. a. Netzelia wailesi. b. Centropyxis aculeata. © Ralf Meisterfeld
Although Arcellinida move and feed with pseudopodia, they are not always easy to see. Especially in soil dwelling species, pseudopodia are difficult to observe. Therfore the kind of pseudopodial movement is not as important for the classification of testate amoebae as it is for naked amoebae. Many species and even genus diagnoses are based on empty tests alone. Most Arcellinida have endolobopodia (Fig. 9), with a hyalin ectoplasma and a more or less granular endoplasma. A second type are the completely hyaline ectoplasmatic reticulolobopodia. These pseudopodia can sometimes form anastomoses and are characteristic for the Phryganellina (Fig. 10). Bonnet (1961, 1963) who has studied the pseudopodia of 17 soil species has found transitions between these two types in Plagiopyxis. The pseudopodial characters change with the kind of activity. Whether the distinction of taxa could be based on the differences between pseudopodia cannot be decided with the data available.



Fig. 10: Reticololobopodia. a. Ectoplasmatic, pointed reticulolobopodia of Phryganella hemisphaerica. b. Reticololobopodia of Cryptodifflugia oviformis. © Ralf Meisterfeld
Symbionts
Several Difflugia, Heleopera and Hyalosphenia species harbour algal symbionts (zoochlorellae). Because the host cells depend on the photosynthesis of their symbionts they are restricted to habitats with sufficient light, like the green parts of wet mosses, periphyton (Aufwuchs) or the surface of sediments, and they are completely absent from soils and deep lake sediments. In the top layer of wet sphagnum mosses the communities of Arcellinida are dominated by "green" Heleopera and Hyalosphenia species.
Reproduction
Arcellinida reproduce by binary fission. The first stage is the construction of an identical daughter shell. Depending on the type of shell, organic building units or mineral particles and cement are arranged around a cytoplasmic extension functioning as a template. On completion of the new test, fission of the nucleus takes place usually as closed orthomitosis. Studies on the ultrastructure of mitosis in the Arcellinida are very scarce (Raikov &. Mignot, 1991) as are those of the morphogenesis of the shell. (Arcella: Netzel, 1975; Netzel & Grunewald, 1977; Mignot & Raikov, 1990. Centropyxis: Netzel, 1972. Heleopera: Ogden, 1989; Netzelia: Netzel, 1983. Phryganella: Ogden & Pitta, 1989.)
Sexual reproduction if present is rare. Mignot & Raikov (1992) have presented details of meiosis and autogamy in the cysts of Arcella. Nuclear division and fusion within cysts are known from Paraquadrula (Lüftenegger & Foissner, 1991) and Heleopera (R. M. pers. observ.).
Cysts
Under unfavourable environmental conditions most Arcellinida produce cysts (Fig. 11).
A few aquatic genera (e.g. many Difflugia) are believed not to form cysts. Resting cysts are formed within the test and have a thick organic wall. Soil and moss species additionally have a special kind of short-term cyst, the precyst. It is characterised by a relative thin membrane enclosing the cell and a diaphragm, which closes the aperture. Often this diaphragm is externally enforced by a detrital plug. Some Microcoryciidae have external cysts.
Ecology
Water availability is the primary factor that determines the distribution of testate amoebae in mosses (Meisterfeld, 1977; 1979b, Tolonen et al,. 1992, Mitchell et al., 1999) and soils and the rate of population turnover (Lousier, 1974; Coûteaux, 1976a, b). Other factors such as organic matter content, pH, and soil nutrient content are also important. Bonnet (e.g. 1964, 1985, 1988 and 1990) has analysed the distribution of testate amoebae in different soil types using multivariate statistics and found a significant correlation between soil or humus type and arcellinid communities.
Similar relations have been found in lake sediments (Schönborn et al., 1966). Mitchell et al., 2004 have shown that the moss elemental chemistry influences the structure of the communities of testate amoebae.
This "bioindicative potential" has been used to monitor the effects of organic and mineral fertilizers (Chardez et al., 1972; Aescht and Foissner, 1992), liming (Wanner, 1991) or organic and conventional farming (Foissner et al., 1987) through changes in testate amoeba community structure.
Their empty tests remain intact after the death of the amoebae and can be determined in most cases to species. In contrast to most other freshwater and soil protozoa, this allows the estimation of more accurate death rates. This hidden mortality reflects a significant part of the population turnover (Lousier, 1974, 1984a, b, 1985, Lousier & Parkinson, 1984).
Testate amoebae are an important component in aquatic and terrestrial ecosystems. In soils they have abundances between 106 and 108 m-2 and annual biomass productions ranging from 1 to more than 200 g (Schönborn, 1977, 1982; Foissner & Adam, 1981; Lousier & Parkinson, 1984; Meisterfeld, 1986, 1989). In many temperate forest soils they are as important as earthworms! They are consumers of bacteria, fungi and different algae groups. Arcellinida may feed selectively. Although some species can perforate fungal mycelia, they usually prefer smaller microbiota (Meisterfeld et al., 1992). Larger Heleoperidae and Nebelidae are predators of small Euglyphida (e.g. Ogden, 1989) and can use the siliceous body plates of the prey to build their own shell. Some species can feed on micro-metazoa such as nematodes (Yeats & Foissner, 1995) and rotifers (Gilbert et al., 2000).
Geographical Distribution
Testate amoebae are found world-wide. But the global diversity of the Arcellinida and the testate amoebae in general is largely unknown. Two thirds of all faunistic records originate from Europe. Whether Arcellinida species have a cosmopolitan distribution is an old but ongoing discussion (Finlay et al. 2004, Foissner, 2006, Mitchell & Meisterfeld 2005). Several very often conspicuous species mainly from the families Arcellidae, Distomatopyxidae, Hyalospheniidae and Lamtopyxidae certainly have a restricted geographical distribution which is not a result of uneven sampling effort.
Fossil Record
Because of the test Arcellinida can fossilize making them interesting candidates for the dating and calibration of the timetable of the tree of life. Recently a diverse assemblage of precambrian (742 million years bp) fossils has been found in the Chuar formation (Grand Canyon), which most likely belong to the Arcellinida (Porter et al., 2003). These are the oldest phagotrophic eukaryotes known today! The resemblance of the precambrian fossils e.g. Paleoarcella athanata Porter, Meisterfeld & Knoll to Arcella hemisphaerica Perty (Fig. 12) is striking! Test morphology seems to be constant over a very long period. Although there will most likely never be definitive proof that these fossils are remains of Arcellinids, this early occurrence is in congruence with the molecular time-scale (Berney & Pawlowski, 2006).

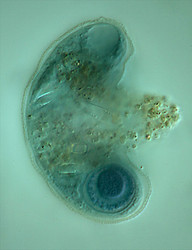
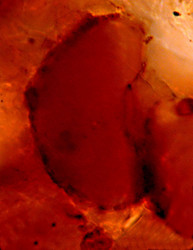
Fig. 12: The recent Arcella hemisphaerica (55 µm) and the precambrian fossil Paleoarcella athanata (40 µm) from the Grand Canyon. © Ralf Meisterfeld
Younger fossils have been described from the Jurassic (Schmidt et al., 2004). The good preservation of the tests of Arcellinida in peat and lake-sediments makes them useful tools in the reconstruction of the paleoenvironments (e.g. Beyens & Meisterfeld, 2001; Charman, 2001, Mitchell et al., 2001, Warner, 1988). Paleoecologists can reconstruct water table changes in bogs, eutrophication processes in lakes or salinity changes in costal wetlands. The global climate change discussion has stimulated the research in this field significantly.
Discussion of Phylogenetic Relationships
Despite their ecological importance the reconstruction of the evolutionary history of the lobose testate amoebae (Arcellinida) is still at its beginning. Until recently phylogenetic relationships among the Arcellinida were based only on morphological criteria, but the morphological approach did not allow a placement of the group in the tree of life. Not until the first partial SSU rRNA sequences were available could the Arcellinida be placed within the Amoebozoa (Nikolaev et al., 2005). This placement has been confirmed by Lara et al., 2008 and Tekle et al., 2008. The monophyly of the Arcellinida is not always supported. In an actin gene tree the Arcellinida are not monophyletic (Tekle et al., 2008).
At the moment molecular analyses suffer from
- insufficient taxon sampling (only a small fraction of genera with mostly only one ore two species)
- very heterogeneous rates of evolution
- problematic alignments of sequences because of very different lengths of the SSU rRNA gene.
In conventional non-phylogeny-based classifications (e.g. Meisterfeld, 2002) the Arcellinida are divided in the three suborders Arcellina (with tree families), Difflugina (with twelve families) and Phryganellina (with two families).
From preliminary analyses of SSU rRNA and actin gene trees one can expect that this structure and the classification of the Arcellinida in general is very likely to be significantly revised as more molecular data become available. Finally, the molecular data also revealed the existence of clear differences in the SSU rRNA gene between even only slightly morphologically distinct taxa, which had been described as “varieties”. This suggests that the diversity of Arcellinida is probably underestimated.
References
General Papers
Meisterfeld, R. 2002. Order Arcellinida Kent. In: An Illustrated Guide to the Protozoa. Lee, J.J.; Leedale, G.F. & Bradbury, P. (eds.). 2nd edition. Soc. of Protozoologists, Lawrence Kansas U.S.A., Allen Press Inc. Vol 2: 827 860.
Meisterfeld, R. 2008. Testate amoebae in mosses and forest soils. In : Röttger, R. A & Foissner, W. 2008. A Course in Protozoology. Shaker Publishing, Aachen, Germany.
Ogden, C. G. & Hedley, R. H. 1980. An Atlas of Freshwater Testate Amoebae. Oxford University Press, Oxford.
Cited Papers
Aescht, E. & Foissner, W. 1992. Effects of Mineral and Organic Fertilizers on the Microfauna in a High-Altitude Reafforestation Trial. Biol. Fertil. Soils, 13:17-24.
Aoki, Y., Hoshino, M. & Matsubara, T. 2007. Silica and testate amoebae in soil under pine-oak forest. Geoderma, 142: 29-35.
Berney, C. & Pawlowski, J. 2006. A molecular time-scale for eukaryote evolution recalibrated with the continuous microfossil record. Proc. R. Soc. B, 273: 1867-1872.
Beyens, L.; Meisterfeld, R. 2001. 7. Protozoa: Testate Amoebae. in: Smol, J.P.; Birks, H.J.B. & Last, W.M. (eds.): Tracking Environmental Change Using Lake Sediments. Vol. 3: Terrestrial, Algal and Siliceous Indicators. Kluwer Acad. Publ., Dordrecht, The Netherlands. Vol 3: 121 153.
Bonnet, L. 1961. L'émission pseudopodique, chez les thécamoebiens endogés (I). Bull. Soc. Zool. France, 86:17-28.
Bonnet, L. 1963. L'émission pseudopodique chez les thécamoebiens endogés. (II) . Bull. Soc. Zool. France, 88:57-63.
Bonnet, L. 1964. Le peuplement thécamoebien des sols. Rev. Ecol. Biol. Sol, 1:123-408.
Bonnet, L. 1985. Le signalement écologique des thécamoebiens des sols. Bull. Soc. Hist. Nat. Toulouse, 121:7-12.
Bonnet, L. 1988. Ecologie du genre Plagiopyxis. (Thécamoebiens des sols). Bull. Soc. Hist. Nat. Toulouse, 124:13-21.
Bonnet, L. 1990. Données écologiques sur quelques Hyalospheniidae et Paraquadrulidae (Thécamoebiens) des sols (Deuxième partie: genre Nebela). Bull. Soc. Hist. Nat. Toulouse, 126:9-17.
Chardez, D., Delecour, F. & Weissen, F. 1972. Evolution des populations thécamoebiennes de sols forestiers sous l'influence de fumures artificielles. Rev. Ecol. Biol. Sol, 9:185-196.
Charman, D. J. 2001. Biostratigraphic and palaeoenvironmental applications of testate amoebae. Quatern. Sci. Rev. 20 (16-17): 1753-1764.
Coûteaux, M.-M. 1976a. Dynamisme de l'équilibre des Thécamoebiens dans quelques sols climaciques. Mem. Mus. Nat. Hist. Nat., Ser. A, Zool., 96:1-183.
Coûteaux, M.-M. 1976b. Le peuplement Thécamoebien du sol et la nature de l'eau disponible. Bull. Ecol., 7:197-206.
Finlay, B.J.; Esteban, G.F. & Fenchel, T. 2004. Protist Diversity is Different? Protist, 155: 15 22.
Foissner, W. & Adam, H. 1981. Die Gemeinschaftsstruktur und Produktion der terricolen Testaceen (Protozoa, Rhizopoda) in einigen Böden der Österreichischen Zentralalpen (Hohe Tauern, Glocknergebiet). Veröff. Österr. MaB-Programms, 4:53-78.
Foissner, W., Franz, H. & Adam, H. 1987. Untersuchungen über das Bodenleben in ökologisch und konventionell bewirtschafteten Acker- und Grünlandböden im Raum Salzburg. Verh. Ges. Ökol. (Graz 1985), 15:333-339.
Foissner, W. 2006. Biogeography and Dispersal of Micro organisms: A Review Emphasizing Protists. Acta Protozoologica, 45, 111 136.
Gilbert, D.; Amblard, C.; Bourdier, G.; Francez, A.J. & Mitchell, E.A.D. 2000. Le régime alimentaire des Thécamoebiens (Protista, Sarcodina). Année Biol., 39: 57- 68.
Harrison, F. W., Dunkelberger, D., Watanabe, N. & Stump, A. B. 1976. The cytology of the testaceous Rhizopod Lesquereusia spiralis (Ehrenberg) Penard. II. Ultrastucture and shell formation. J. Morph., 150:343-358.
Harrison, F. W., Dunkelberger, D., Watanabe, N. & Stump, A. B. 1981. Ultrastructure and deposition of silica in rhizopod amoebae. In: Simpson, T.L., Volcani, B.E., (eds.), Silicon and Siliceous Structures in Biological Systems, Springer-Verlag, New York: 281-294.
Lara, E.; Heger, T.J.; Ekelund, F.; Lamentowicz, M. & Mitchell, E.A.D. 2008, Ribosomal RNA Genes Challenge the Monophyly of the Hyalospheniidae (Amoebozoa: Arcellinida). Protist, 159: 165 176.
Lousier, J. D. 1974. Effects of experimental soil moisture fluctuations on turnover-rates of Testacea. Soil Biol. Biochem., 6:19-26.
Lousier, J. D. 1984a. Population dynamics and production studies of Phryganella acropodia and Difflugiella oviformis (Testacea, Rhizopoda, Protozoa) in an Aspen woodland soil. Pedobiologia, 26:331-347.
Lousier, J. D. 1984b. Population dynamics and production studies of species of Nebelidae (Testacea, Rhizopoda) in an Aspen woodland soil. Acta Protozool., 23: 145-159.
Lousier, J. D. 1985. Population dynamics and production studies of species of Centropyxidae (Testacea, Rhizopoda) in an Aspen woodland soil. Arch. Protistenk., 130:165-178.
Lousier, J. D. & Parkinson, D. 1984. Annual population dynamics and production ecology of Testacea (Rhizopoda Protozoa) in an Aspen woodland soil. Soil Biol. Biochem., 16:103-114.
Lüftenegger, G. & Foissner, W. 1991. Morphology and biometry of twelve soil testate amoebae (Protozoa, Rhizopoda) from Australia, Africa, and Austria. Bull. Br. Mus. nat. Hist. (Zool.), 57:1-16.
Meisterfeld, R. 1977. Die horizontale und vertikale Verteilung der Testaceen (Rhizopoda, Testacea) in Sphagnum. Arch. Hydrobiol., 79:319-356.
Meisterfeld, R. 1978. Die Struktur von Testaceenzönosen (Rhizopoda, Testacea) in Sphagnum unter besonderer Berücksichtigung ihrer Diversität. Verh. Ges. Ökol. (Kiel 1977), 7:441-450.
Meisterfeld, R. 1979. Clusteranalytische Differenzierung der Testaceenzönosen (Rhizopoda, Testacea) in Sphagnum. Arch. Protistenk., 121:270-307.
Meisterfeld, R. 1986. The importance of Protozoa in a Beech Forest Ecosystem. Symp. Biol. Hung., 33:291-299.
Meisterfeld, R. 1989. Die Bedeutung der Protozoen im Kohlenstoffhaushalt eines Kalkbuchenwaldes. (Zur Funktion der Fauna in einem Mullbuchenwald 3). Verh. Ges. Ökol. (Göttingen 1987), 17:221-227.
Meisterfeld, R. 2002. Order Arcellinida Kent. In: An Illustrated Guide to the Protozoa. Lee, J.J.; Leedale, G.F. & Bradbury, P. (eds.). 2nd edition. Soc. of Protozoologists, Lawrence Kansas U.S.A., Allen Press Inc. Vol 2: 827 860.
Meisterfeld, R., Dohmen, C., Meyer, A., Panfil, C. & Wienand, J. 1992. Influence of Food Quality and Quantity on Growth and Feeding Rates of Testate Amoebae (Testacealobosia). Europ. J. Protistol., 28:351.
Mignot, J. P. & Raikov, I. B. 1990. New Ultrastructural Data on the Morphogenesis of the Test in the Testacea Arcella vulgaris. Europ. J. Protistol., 26:132-141.
Mignot, J. P. & Raikov, I. B. 1992. Evidence for Meiosis in the Testate Amoeba Arcella. J. Protozool., 39:287-289.
Mitchell, E.A.D.; Buttler, A.J.; Warner, B.G. & Gobat, J.M. 1999. Ecology of testate amoebae (Protozoa: Rhizopoda) in Sphagnum peatlands in the Jura mountains, Switzerland and France. Ecoscience. 6 (4): 565 576.
Mitchell, E. A. D.; van der Knaap, W.O.; van Leeuwen, J. F. N.; Buttler, A.; Warner, B. G. and Gobat, J.M. 2001. The palaeoecological history of the Praz-Rodet bog (Swiss Jura) based on pollen, plant macrofossils and testate amoebae (Protozoa). Holocene 11: 65-80.
Mitchell, E.A.D.; Gilbert, D.; Buttler, A.; Amblard, C.; Grosvernier, P. & Gobat, J.M. 2003. Structure of microbial communities in Sphagnum peatlands and effect of atmospheric carbon dioxide enrichment. Microbial Ecology 46: 187-199.
Mitchell, E.A.D.; Bragazza, L. & Gerdol, R. 2004. Testate amoebae (Protista) communities in Hylocomium splendens (Hedw.) BSG (Bryophyta): Relationships with altitude, and moss elemental chemistry. Protist, 155: 423-436.
Mitchell, E.A.D. and Meisterfeld, R. 2005. Taxonomic Confusion Blurs the Debate on Cosmopolitanism versus Local Endemism of Free Living Protists. Protist, 156: 263-267.
Netzel, H. 1972. Die Bildung der Gehäusewand bei der Thekamöbe Centropyxis discoides (Rhizopoda, Testacea). Z. Zellforschg., 135:45-54.
Netzel, H. 1975. Die Entstehung der hexagonalen Schalenstruktur bei der Thekamöbe Arcella vulgaris var. multinucleata (Rhizopoda, Testacea). Arch. Protistenk., 117:321-357.
Netzel, H. 1983. Gehäusewandbildung durch mehrphasige Sekretion bei der Thekamöbe Netzelia oviformis (Rhizopoda Testacea). Arch. Protistenk.,127:351-381.
Netzel, H. & Grunewald, B. 1977. Morphogenesis in the shelled rhizopod Arcella dentata. Protistologica, 13:299-319.
Nikolaev, S.I.; Mitchell, E.A.D.; Petrov, N.B.; Berney, C.; Fahrni, J.; Pawlowski, J. 2005. The Testate Lobobose Amoebae (Order Arcellinida Kent, 1880) Finally Find their Home within Amoebozoa. Protist, 156: 191 202.
Ogden, C. G. 1989. The agglutinate shell of Heleopera petricola (Protozoa, Rhizopoda), factors affecting its structure and composition. Arch. Protistenk., 137:9-24.
Ogden, C. G. 1990. The structure of the shell wall in testate amoebae and the importance of the organic cement matrix. in: Scanning Electron Microscopy in Taxonomy and Functional Morphology. Ed.: Claugher, D. Syst. Ass. Special Vol., Clarendon Press, Oxford, 41:235-257.
Ogden, C. G. & Hedley, R. H. 1980. An Atlas of Freshwater Testate Amoebae. Oxford University Press, Oxford.
Ogden, C. G. & Pitta, P. 1989. Morphology and construction of the shell wall in an agglutinate soil testate amoeba Phryganella acropodia (Rhizopoda). J. Protozool., 36:437-445.
Porter, S.M., Meisterfeld, R. and Knoll, A.H. 2003. Vase shaped microfossils from the neoproterozoic Chuar Group, Grand Canyon: A classification guided by modern testate amoebae. J. Paleont. 77: 409-429.
Raikov, I. B. 1982. The Protozoan Nucleus. Morphology and Evolution. Springer-Verlag, Berlin.
Raikov, I. B., Karadzhan, B. P., Kaur, R. & Mignot, J. P. 1989. Nuclear fine structure at interphase and during encystment in 2 forms of the testacea Arcella vulgaris. Europ. J. Protistol., 24:369-380.
Raikov, I. B. & Mignot, J. P. 1991. Fine-Structural Study of Mitosis in the Testacea Arcella vulgaris Ehrbg. Europ. J. Protistol., 26:340-349.
Schmidt, A.R.; Schönborn, W. and Schäfer, U. 2004. Diverse Fossil Amoebae in German Mesozoic Amber. Palaeontol. 47 (Part 2): 185-197, 1 plate.
Schönborn, W. 1977. Production studies on Protozoa. Oecologia, 27:171-184.
Schönborn, W. 1982. Estimation of annual production of Testacea (Protozoa) in mull and moder (II). Pedobiologia, 23:383-393.
Schönborn, W., Flössner, D. & Proft, G. 1966. Die limnologische Charakterisierung des Profundals einiger norddeutscher und masurischer Seen mit Hilfe von Testaceen-Gemeinschaften. Verh. Int. Verein. Limnol., 16:251-257.
Schröter, D.; Wolters, V.; De Ruiter, P.C. 2003. C and N mineralisation in the decomposer food webs of a European forest transect. Oikos. 102: 294 308.
Tolonen, K., Warner, B. G. & Vasander, H. 1992. Ecology of Testacea (Protozoa, Rhizopoda) in Mires in Southern Finland. 1. Autecology. Arch. Protistenk., 142:119-138.
Wanner, M. 1991. Zur Ökologie von Thekamöben (Protozoa: Rhizopoda) in süd-deutschen Wäldern. Arch. Protistenk., 140:237-288.
Warner, B.G. 1988. Methods in Quarternary Ecology. 5. Testate amoebae (Protozoa). Geoscience Canada 15: 251-260.
Yeates, G.W. & Foissner, W. 1995. Testate amoebae as predators of nematodes. Biol. Fertil. Soils, 20: 1-7.
Title Illustrations

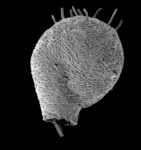
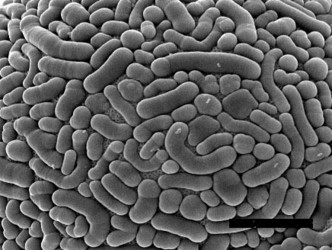
| Scientific Name | Amphizonella violacea |
|---|---|
| Location | Aachen, Germany |
| Specimen Condition | Live Specimen |
| Identified By | Ralf Meisterfeld |
| Life Cycle Stage | vegetative |
| Size | 170 µm |
| Copyright |
©

|
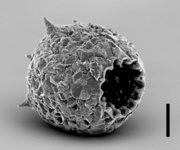
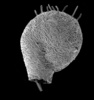
| Scientific Name | Arcella dentata |
|---|---|
| Specimen Condition | empty test |
| Identified By | Ralf Meisterfeld |
| Copyright |
© 2000

|
| Scientific Name | Lesquereusia spiralis hirsuta |
|---|---|
| Location | Tully, Queensland, Australia |
| Specimen Condition | empty test |
| Identified By | Ralf Meisterfeld |
| Copyright |
©

|
| Scientific Name | Difflugia corona |
|---|---|
| Location | Pond near Aachen, Germany |
| Identified By | Ralf Meisterfeld |
| Copyright |
©

|
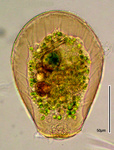
| Scientific Name | Hyalosphenia papilio |
|---|---|
| Identified By | Edward Mitchell |
| Copyright |
© Edward Mitchell

|
About This Page
This page is being developed as part of the Tree of Life Web Project Protist Diversity Workshop, co-sponsored by the Canadian Institute for Advanced Research (CIFAR) program in Integrated Microbial Biodiversity and the Tula Foundation.

Rheinisch-Westfälische Technische Hochschule Aachen, Germany
Edward Mitchell

University of Neuchâtel
Correspondence regarding this page should be directed to Ralf Meisterfeld at and Edward Mitchell at
Page copyright © 2008 and Edward Mitchell
All Rights Reserved.
- First online 02 September 2008
- Content changed 02 September 2008
Citing this page:
Meisterfeld, Ralf and Edward Mitchell. 2008. Arcellinida . Testate Lobose Amoebae. Version 02 September 2008 (under construction). http://tolweb.org/Arcellinida/124471/2008.09.02 in The Tree of Life Web Project, http://tolweb.org/














 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site