Chaetothyriales
Cecile Gueidan- Chaetothyriaceae
- Herpotrichiellaceae
Introduction
The order Chaetothyriales includes non-lichenized ascomycetes divided in two different families, the Chaetothyriaceae and the Herpotrichiellaceae (Geiser et al. 2006). The Chaeothyriaceae are known as epiphytes, colonizing the leaves and the bark of trees in the Tropics (Batista and Ciferri 1962). For many of these species of Chaetothyriaceae, it is still unclear whether they are saprophytic or biotrophic (Barr 1987). Species of the Herpotrichiellaceae are known either from their sexually reproducing stage (teleomorph), their clonally reproducing stage (anamorph), or both. Teleomorphs include small inconspicuous saprophytes, mainly growing on decaying wood and mushrooms (Barr 1987; Untereiner and Naveau 1999). Anamorphs are found as animal and human opportunistic pathogens, or are sometimes isolated from the environment using enrichment methods (Prenafeta-Boldú et al. 2006). The opportunistic pathogens, also called black yeasts, can induce skin and nervous system infections in healthy or immunocompromised patients (de Hoog et al 2000; Horré and de Hoog 1999; Kantarcioglu and de Hoog 2004), and their addition to the Eurotiomycetes places them with most other animal pathogens in the Pezizomycotina. Most of these species were only known in their anamorphic stages, and it is only recently, with the development of molecular techniques, that the connections between anamorphs and teleomorphs were assessed (Untereiner 1997, 2000; Untereiner and Naveau 1999; Untereiner et al 2002). Because little is known on the ecology of these organisms, the study of anamorph/teleomorph relationships and the isolation of strains in their putative primary habitats are a key issue to the understanding of the evolution of this group (de Hoog et al. 2000).
Recent studies showed that some slow-growing melanized fungi inhabiting rocks in harsh environments, belong to the Chaetothyriales (Ruibal 2004; Sterflinger et al 1999). These fungi were qualified as extremotolerant, because they are able to tolerate extreme conditions, such as very high or very low temperatures, osmotic stress, dessication, and irradiation. This extremotolerance is conferred to them by adaptations, either morphological (melanization, meristematic growth, endoconidiation) or physiological (oligotrophism, slow metabolism). Molecular data suggest that they form novel lineages within Chaetothyriales and indicate that more diversity is yet to be discovered for these rock-inhabiting species (Ruibal 2004).
Characteristics
The order Chaetothyriales is characterized by a dark mycelium, growing as a loose net of hyphae (mycelial pellicle) over the substrate in the Chaetothyriaceae (Batista & Ciferri 1962), or as inconspicuous immersed mycelium in the teleomorphs of Herpotrichiellaceae (Untereiner 2000). Meristematic and yeast-like growths are also very common in Herpotrichiellaceae. The dark coloration of the mycelium is due to the production of a melanin pigment. This pigment was shown to contribute to the resistance of these fungi to host immune responses (Schnitzler et al. 1999). However, the presence of melanin is not sufficient to explain pathogenicity, and additional factors must be involved to explain the virulence of these fungi (de Hoog et al. 2000).
The ascomata of Chaetothyriales tend to be inconspicuous and ephemeral, explaining why this group, although common in nature, has been overlooked by collectors. The perithecial ascomata are erumpent or superficial, sometimes setose, with or without a periphysate ostiole (Kirk et al. 2001). The excipulum pigmentation is variable, and the wall is thin and pseudoparenchymatous. The hamathecium consists of short apical periphysoids. Asci are clavate, with a fissitunicate mode of dehiscence and a thickening in the apical region. Spores are hyaline to pale grey, and transversally septate to muriform.
Discussion of Phylogenetic Relationships
Because little is known about the family Chaetothyriaceae and molecular data lack for this family (except for the genus Ceramothyrium), its relationship with other members of the Chaetothyriales is still unclear. Within the Herpotrichiellaceae, the generic delimitation is really problematic, mostly due to the numerous anamorphic genera (i.e., Cladophialophora, Ramichloridium, Phialophora, Exophiala), which appeared to be highly polyphyletic (Haase et al. 1999). Adding to that, the teleomorphic genus Capronia also appeared to be polyphyletic (Untereiner 2000).
References
Barr 1987. New taxa and combinations in the Loculoascomycetes. Mycotaxon 29: 501-505.
Batista AC, Ciferri R. 1962. The Chaetothyriales. Sydowia 3: 1-129.
Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, Hofstetter V, Fraker E, Schoch C, Tibell L, Untereiner WA, Aptroot A. 2006. Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98: 1054-1065.
Haase G, Sonntag L, Melzer-Krick B, de Hoog GS. 1999. Phylogenetic inference by SSU gene analysis of members of the Herpotrichiellaceae, with special reference to human pathogenic species. Studies in Mycology 43: 80-97.
Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, T. James, P. M. Kirk, R. Lücking, T. Lumbsch, F. Lutzoni, P. B. Matheny, D. J. Mclaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y.-C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, C. Gueidan, D. L. Hawksworth, G. Hestmark, K. Hosaka, R. A. Humber, K. Hyde, J. E. Ironside, U. Kõljalg, C. P. Kurtzman, K.-H. Larsson, R. Lichtwardt, J. Longcore, J. Miądlikowska, A. Miller, J.-M. Moncalvo, S. Mozley-Standridge, F. Oberwinkler, E. Parmasto, V. Reeb, J. D. Rogers, C. Roux, L. Ryvarden, J. P. Sampaio, A. Schüßler, J. Sugiyama, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiß, M. M. White, K. Winka, Y.-J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509-547.
de Hoog GS, Queiroz-Telles F, Haase G, Fernadez-Zeppenfeldt G, Angelis DA, van den Ende A, Matos T, Peltroche-Llacsahuanga H, Pizzirani-Kleiner AA, Rainer J, Richard-Yegres N, Vicente V, Yegres F. 2000. Black fungi: clinical and pathogenic approaches. Medical Mycology 38: 243-250.
Horré R, de Hoog GS. 1999. Primary cerebral infections by melanized fungi: a review. Studies in Mycology 43: 176-193.
Kantarcioglu AS, de Hoog GS. 2004. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses 47: 5-13.
Kirk PM, David JC, Stalpers JA. 2001. Ainsworth & Bisby's Dictionary of the Fungi. Wallingford, UK: CAB International. 650 p.
Prenafeta-Boldú, FX, Summerbell, R, de Hoog, GS. 2006. Fungi growing on aromatic hydrocarbons: biotechnology’s unexpected encounter with biohazard? FEMS Microbiol Rev 30:109-130.
Ruibal C. 2004. Isolation and characterization of melanized, slow-growing fungi from semiarid rock surfaces of cental Spain and Mallorca. pp. 158. Universidad Automoma de Madríd, Madríd.
Schnitzler N, Peltroche-Llacsahuanga H, Bestier N, Zijndorf J, Lütticken R, Haase G. 1999. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infection and Immunity 67: 94-101.
Sterflinger K, de Hoog GS, Haase G. 1999. Phylogeny and ecology of meristematic ascomycetes. Studies in Mycology 43: 5-22.
Untereiner WA. 1997. Taxonomy of selected members of the ascomycete genus Capronia with notes on anamorph-teleomorph connections. Mycologia 89: 120-131.
Untereiner WA. 2000. Capronia and its anamorphs: exploring the value of morphological and molecular characteris in the systematics of the Herpotrichiellaceae. Studies in Mycology 45: 141-148.
Untereiner WA, Naveau FA. 1999. Molecular systematics of the Herpotrichiellaceae, with an assessment of the phylogenetic positions of Exophiala dermatitidis and Phialophora americana. Mycologia 91: 67-83.
Untereiner WA, Scott JA, Naveau FA, Bachewich J. 2002. Phylogeny of Ajellomyces, Polytolypa and Spiromastix (Onygenaceae) inferred from rDNA sequence and non-molecular data. Studies in Mycology: 25-35.
Title Illustrations

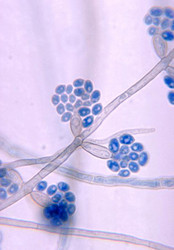
| Scientific Name | Exophiala salmonis |
|---|---|
| Body Part | conidiophores |
| Source Collection | Public Health Image Library (Centers for Disease Control) |
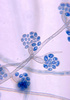
| Scientific Name | Cladophialophora carrionii |
|---|---|
| Creator | CDC/Dr. Lucille K. Georg |
| Source Collection | Public Health Image Library (Centers for Disease Control) |
About This Page

Duke University, Durham, North Carolina, USA
Correspondence regarding this page should be directed to Cecile Gueidan at
Page copyright © 2008
All Rights Reserved.
- First online 21 December 2007
- Content changed 29 January 2008
Citing this page:
Gueidan, Cecile. 2008. Chaetothyriales. Version 29 January 2008 (under construction). http://tolweb.org/Chaetothyriales/29305/2008.01.29 in The Tree of Life Web Project, http://tolweb.org/








 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site